Dr. NPK of Elite Garden Wholesale takes us through why you should test your runoff nutrient solution, and what some of your results might mean.

The following is an article produced by a contributing author. Growers Network does not endorse nor evaluate the claims of our contributors, nor do they influence our editorial process. We thank our contributors for their time and effort so we can continue our exclusive Growers Spotlight service.
Disclaimer
This article has been republished with permission from Elite Garden Wholesale. The original article can be found here.
The cannabis industry has all sorts of different jobs. Whether you’re growing, processing, selling, or consuming, we all play a different role, and every role is important. As growers, we can be “field scientists,” since we are creating products for other people to use.
Sometimes we can forget that creation is not the only thing that matters; the quality of our creations is equally as important! To that end, we want to help growers learn to use the analytical resources available to them. Today’s blog is about some analytical methods that can be used to test the nutrient concentration of your feeding water, how your plants are using your nutrients, and how that information can help you grow better bud!
PPM and the Elements: Why should I care?
PPM stands for “parts per million” and is a unit of concentration. For example: let’s say there are 3 lbs of cannabis “diluted” in 999,997 lbs of water (for a total of 1 million even), we would say the concentration of cannabis is 3 PPM for those given weights. Another unit of measurement, ppb, is similar in principle but stands for “parts per billion.” When I am referring to PPM, I am referring to the Total Dissolved Solids (or TDS) in a solution. Another measurement that people frequently use is Electrical Conductivity (EC). The working principle is the same, but the measurement is different. Essentially, the more nutrients you have in your water (a higher concentration, higher PPM), the higher your EC is because the nutrients are ionic solids.
Editor’s Note: The correlation of EC and PPM is not linear, and conductivity can also change based on temperature (while PPM will not). The two measurements measure similar things, but are not equivalent measurements.
When we are measuring PPM (usually with the same instrument that measures pH), we are analyzing the total amount of salts and nutrients in the water. This is important because we must make sure that we are adding the right amount of nutrients every feed. Let’s say you measure your reservoir on Tuesday and you get 1,500 PPM. You measure on Wednesday using the same feed and get 2,200 PPM. This can help you recognize that too many nutrients have been added and that your feeding regimen may need to change.
But this isn’t the only reason to measure PPM! Comparing your runoff (the water stream beyond the root zone) to your reservoir provides useful pieces of information that can make sure your plants are performing the best that they can.
How to Measure Your Nutrient Concentration
Every time you take a measurement, whether it be pH, PPM, or EC, you are conducting a science experiment. Like all science experiments, you must make sure they are done properly to prevent errors. Measuring runoff by itself is useless without several additional measurements.
I strongly recommend measuring two things in your water stream: PPM and pH. Both should be measured in your sump and in your runoff. By measuring at a minimum of these two places, you can see how your plants are behaving and potentially taking nutrients.
Editor’s Note: As a scientist myself, I also recommend you take several measurements at the same points in your reservoir and sump -- sometimes a nutrient solution may not be mixed evenly or the runoff might not be mixed evenly, so several readings can help reduce statistical anomalies. We don’t want to jump to conclusions based on the initial findings!
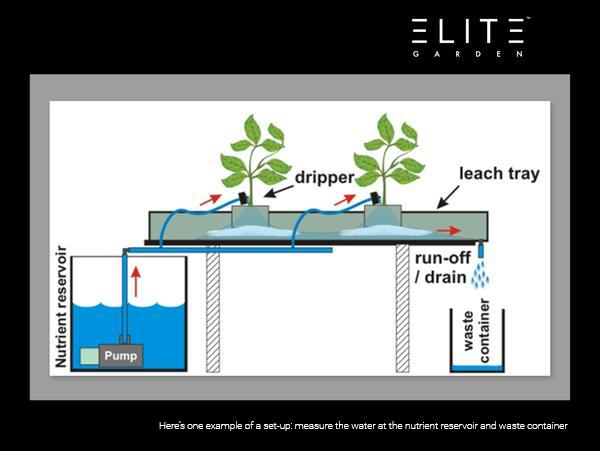
How to Decipher (Some of) the Results
Below I will go over some potential situations that can arise from the analytical results you might get. This is assuming you have already collected a sample/measurement in your sump and your runoff.
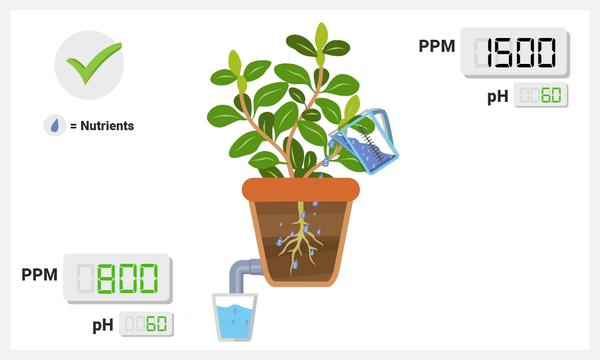
PPM Lower in Runoff than in Sump
This is a good thing! This implies that your plants are taking up nutrients, thus lowering the overall ionic concentration! If you monitor this routinely and you see a standard drop (e.g., 1600 in, 800 out, 6 days a week), that implies that your plants are experiencing roughly the same conditions on a daily level. Keep in mind: if your PPM’s are super low in the runoff, consider upping your nutrient treat rates slowly, as your plants may be lacking in nutrients.
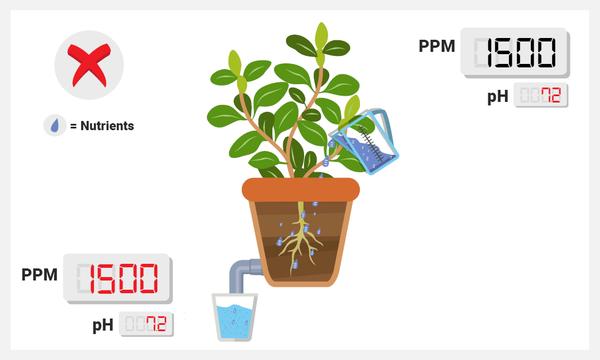
No PPM change between runoff and sump (or lower than normal drop).
This implies that your plants are not properly taking up the nutrients in the sump. One reason for this may be due to pH; certain ions (such as nitrate or phosphate) can only be taken up by the plant in certain ranges.
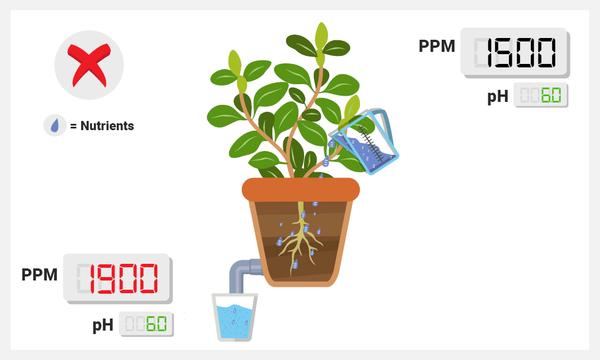
PPM is Lower in Sump than Normal
Check for a white precipitate (a white, powdery-looking solid) in your sump. If you’re sure you added the right amount of nutrients, the PPM might be lower due to nutrient lockout: not all the ions in your sump might be getting along and they may react with one another and form a solid that is not soluble in water (insoluble). One of the most classic culprits is calcium phosphate (from Ca2+ and PO43-). You can help avoid this by practicing proper mixing techniques (see video below).

PPM is Higher in your Runoff than Sump.
This should be rare, and it might indicate some sort of salt buildup at the root zone. This buildup persists for a while, and then slowly dissolves back into the runoff, resulting in a higher PPM. The most common salts that are present in the root zone that are not water soluble are calcium-type salts (calcium phosphate, calcium carbonate, calcium hydroxide). Use a line cleaner, or if you’re at the end of the run and the rinse won’t hit the plants, try dilute muriatic acid to help remove the salt buildup. Copious flushing with water will help too; it’s just a slower process.
Yellowing in the Plants
Look at the elemental values in your runoff compared to your sump. First, ensure that your nitrates (plus total nitrogen), phosphates, and potassium ions are decreasing between your sump and runoff. This shows that the yellowing is not due to a macronutrient deficiency. Continue looking at the other elements to potentially find which element needs to be fortified. If your PPM isn’t lower in your runoff compared to your sump, there are likely some issues with your pH that may also be leading to the yellowing.
pH Increase/Decrease Between Sump and Runoff
A little variance is normal, but a large change is known as pH drift. Keep an eye out for nutrient lockout, as this is one of the main reasons for a significant pH drift. It’s a good idea to monitor this because if your pH is drifting, certain nutrients may not be available to your plants as the pH drifts outside the acceptable uptake range.
Closing Thoughts
Analytical data for your grow is extremely important! Make sure that you always have at least two pieces of data before trying to make a conclusion:
- Concentration (whether it be via PPM or EC)
- pH
These are crucial tools to analyze in both your runoff and sump. It can keep your plants alive and happy!
10 Best Gift Ideas for Cannabis Connoisseurs and Growing Aficionados (2022)
December 7, 2022Developing and Optimizing a Cannabis Cultivation System
December 14, 2021Dealing with Insomnia: How Can CBD Help?
December 10, 2020Your Guide to Sleep and CBD
December 7, 2020
Do you want to receive the next Grower's Spotlight as soon as it's available? Sign up below!
Resources:
Want to get in touch with Elite Garden Wholesale? They can be reached via the following methods:
- Website: https://www.elitegardenwholesale.com/
- Email: info@elitegardenwholesale.com

Do you have any questions or comments?

About the Author
Dr. NPK has been in the chemical formulating business for over 9 years. With a Bachelors in chemistry from UCLA and a Ph.D. in chemistry from UC Irvine, he has always had a passion for chemical formulation. Over the past several years, his focus has narrowed towards the research and development of products that are optimized for cannabis. He assisted in the development of the Elite Nutrient line and takes great pride in the products he created and uses himself. He has made it his mission to cut through all the pseudoscience available on the web and to educate readers on the science behind growing top-shelf bud.